Traditional treatment strategies and their limitations in hair restoration
Traditional treatment strategies have been mainly limited to pharmaceutical and surgical modalities. Currently approved medications, such as Finasteride and Minoxidil, require a great degree of compliance for long periods with varying degrees of effectiveness. Side effects are also common and often dissuade use, including sexual dysfunction, mood disorders, increased prostate/breast cancer risks, and birth defects. Once limited to scalp reduction and rotational flap techniques, surgical treatments have evolved to follicular unit isolation/extraction with punch biopsies or donor strip harvesting. Success and efficiency are affected by the surgeon’s skill set, however, because follicle transection rates and operative speed are limiting factors for both physician and patient. In addition, scars, poor wound healing, and an unnatural hairline are frequently reported concerns and considerations in the hands of less experienced surgeons.The potential of PRP therapy as a “biologically oriented” approach
Recent discoveries in the molecular pathways of the hair cycle, however, have provided the foundation for novel investigations in a “biologically oriented” approach to cell-based therapies in hair restoration.The use of platelet concentrates in regenerative medicine has long been investigated in several surgical and clinical fields. The effects of these concentrates on bone grafting and wound healing have been demonstrated in oral and maxillofacial, orthopedic, and cardiac surgery. More recently, interest has been increasing in dermatology and plastic surgery in both fat grafting and skin rejuvenation.
Essential to wound repair and the inflammatory/remodeling pathway, platelets contain several chemotactic and mitogenic proteins that are released upon activation; growth hormones and cytokines are among the most prominent biologically active and secreted components. Platelet-rich plasma (PRP) is an autogenous, liquid platelet concentrate extracted from a patient’s peripheral blood by centrifugation. Because PRP is autologous, the risk of hypersensitivity or immunogenic reactions and disease transmission is significantly reduced.
The platelet and plasma growth factors isolated in the PRP slurry are in higher concentration per volume, when compared with a patient’s whole blood. The platelet solution may then be activated in vitro (turning to a gelatinous state) or injected in liquid form for activation in vivo. Once activated, platelets release alpha granules containing a myriad of growth factors, including transforming growth factor-ß (TGF-ß), epidermal growth factor (EGF), essential fibroblast growth factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and insulin-like growth factor-1 (IGF-1). These factors have been found to substantially affect the hair cell growth cycle: stimulating differentiation proliferation and hair follicle growth. In brief, the hair cycle consists of a resting telogen phase, an active anagen-growing phase, and an apoptotic catagen phase. Aberrancies in cycling these phases lead to a disruption in hair follicle growth and hair loss. Bulge cells, inducible stem cells found along the hair follicle shaft, have been found to repopulate the hair follicle epithelium and are fundamental to the progression of hair cycling. It is these hair follicles stem cells that contain growth factor receptors responsible for hair growth manipulation and molecular pathway regulation.
Laboratory tests and exclusion criteria for PRP therapy
Laboratory tests to aid with diagnosis include complete blood cell count; and measurement of serum levels of iron, serum ferritin, total iron-binding capacity, folic acid, triiodothyronine, thyroxine, thyroid-stimulating hormone, antithyroid peroxidase, dehydroepiandrosterone, testosterone, prolactin, follicle-stimulating hormone, and luteinizing hormone. Of note, medication history known to affect the cyclooxygenase inflammatory pathway should be documented; these include nonsteroidal anti-inflammatory drugs such as aspirin, glucocorticoids, and immunomodulating therapies.Contraindications and exclusion criteria in several studies include coagulation disorders, platelet dysfunction, anticoagulant therapy, thrombocytopenia, hemodynamic instability, local infection at the site of blood harvest or graft injection, hepatitis, and patients who are prone to keloid formation.
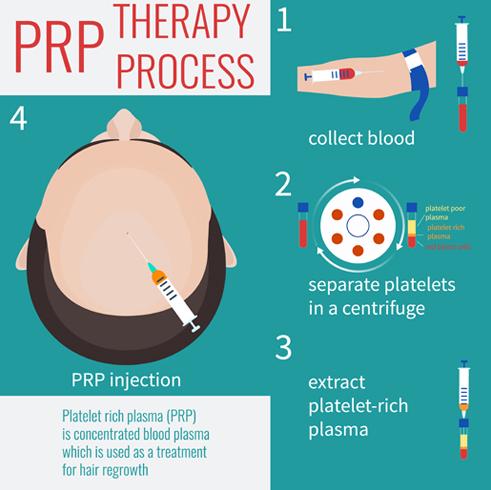
The production of platelet concentrates for PRP therapy
The production of platelet concentrates for platelet-rich solutions begins with the harvest of peripheral venous blood. Care is taken to reduce platelet injury/fragmentation because mechanical trauma may result in unintentional activation; these measures include using large-bore needles and single needle-stick venipuncture attempts. The collected specimen can be centrifuged in 1 or 2 steps depending on the preparation system. The initial, low-force centrifugation allows the blood components to separate into 3 weight-dependent layers:- A top, supernatant, layer of platelet-poor plasma (PPP);
- A “buffy coat” (BC) middle layer rich in platelets and containing white blood cells;
- A bottom, red blood cell (RBC) layer
The US regulatory process for blood products is described in the Food and Drug Administration’s (FDA) 21 CFR 127 of the Code of Regulations73, under the prevue of the FDA’s Center for Biologics Evaluation and Research.
Kolors offers RICH PRP (30 ml) Tricel PRP FDA-approved technique.
- We follow PROCEDURE in OT with the complete sterile medium for procedures.
- Our expert surgeons have over 3 years of experience and are trained in-house for dealing with all sorts of related conditions.
- Centrifuge machine used to activate the platelets is US-FDA approved Tricel PRP (30ml).
- No infection, allergies or side effects are seen in this treatment at Kolors.
Cosmetic Hair Replacement
A tailor-made cosmetic hair replacement technique for the advanced grade of baldness customized to suit individuals’ age, personality and profession to give a head full of hair in just 3 hours.Indications
- ADVANCED GRADES of Androgenetic Alopecia in Men and woman GRADE:-D,E,F OR (5.6,7)
- ALOPECIA TOTALIS
- ALOPECIA UNIVERSALIS
Why Kolors
- Kolors is unique for Tailor-made Cosmetic hair replacement.
- 100% Human Virgin Hair is used.
- Natural look can wash, swim, gym, gel, and style your hair as before.
- Full head of hair in just 3HOURS!!
- A patch of Human virgin hair for covering a bald spot.
- Scalp skin is prepared with 100% human virgin hair exactly matching the color, and the texture of clients’ hair, and this skin is fused to the bald area so that the new hair blends well with the existing hair and gives a natural look.
Methods of fixation
Grip and Strip method

Advantages of CHR
- The scalp patch blends exactly with the existing hair giving a natural look.
- The person with CHR can
- SWIM
- GYM
- TRAVEL
- STYLE&GEL their hair as they used to do it before.







